Endoscopes are essential visualisation tools in modern medicine. Due to cleanliness concerns with flexible devices, single-use endoscopes are beginning to play an increasing role in this space. However, several hurdles to further market penetration remain, such as product cost and wastage. Furthermore, there is an increasing push in endoscope design towards miniaturisation as a means to improve ease of use and reduce patient trauma following interventional procedures and surgery which requires cost/size/performance tradeoffs to be considered.
In order to explore the technological and logistical challenges associated with designing systems that address both these aspects, the medical team at Sagentia conducted an internal project to develop a miniaturised sub-1.5mm scope tip using off-the-shelf hardware. This was done by considering possible illumination and imaging options based on size constraints, as well as designing the assembly and mounting while considering availability and costs. While the process does not have significant technical complexity it is limited by the technological performance of existing hardware and the challenge lies in assembling at such small scales. To that end, the modularity of parts being distributed by the likes of OmniVision and Schott AG is of benefit. One player in the space of single-use endoscopes is Ambu A/S, which plans to release single-use colonoscopes and gastroscopes in 2021.
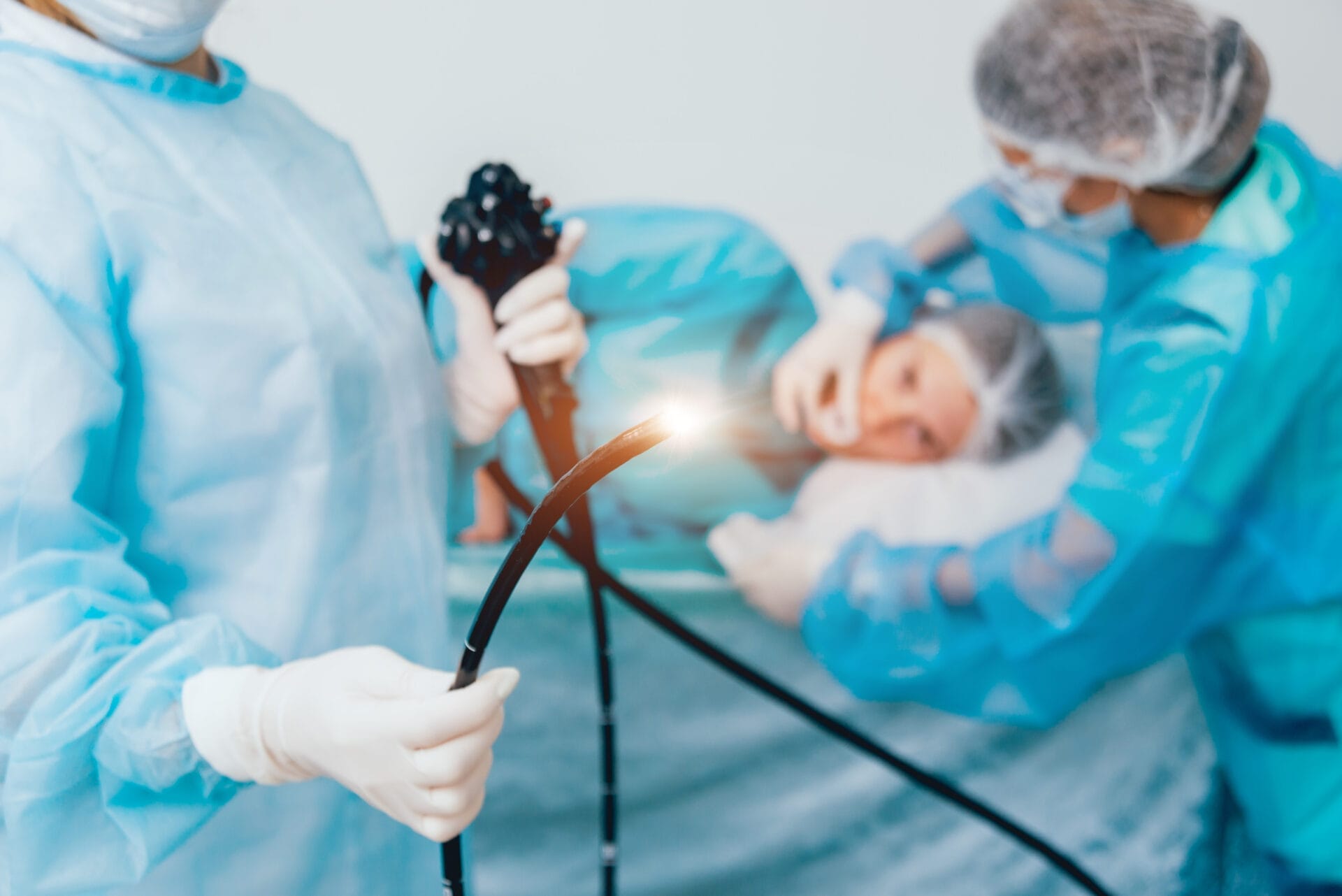
However, some reusable endoscopes may suffer from issues with cleanliness between surgical procedures. According to the American Journal of Infection Control, as many as 71% of reprocessed endoscopes can contain some form of microbial growth, leading to potentially severe complications that can ultimately compromise patient health. The problem mostly affects procedures involving flexible endoscopes (see Figure 1), which generally do not undergo the high temperatures of a steam sterilisation process, instead being suitable for only high-level disinfection. In particular, in 2013 the Centers for Disease Control and Prevention (CDC) found a potential association between multi-drug resistant bacteria and improper cleaning of flexible duodenoscopes. The FDA has issued warning letters to several endoscope manufacturers as a result.
Single-use flexible endoscopes have emerged as a possible solution to this issue. This white paper discusses:
- The endoscope market
- Disposable endoscope design
- Challenges and solutions for single-use endoscopes
- The future for single-use flexible endoscopy
Download the full white paper below
Read more of our insights


June 20, 2025
Closing the Alzheimer’s gender gap: innovating around early diagnosis and treatment

April 24, 2025
Overcoming technical barriers to blood-based diagnosis of neurodegenerative disease

February 13, 2025
Five critical factors for the commercialisation of women’s health technology