ECHA recommends four new substances for REACH authorisation, including melamine. What does this mean for industry?
November 21, 2025

The European Chemicals Agency (ECHA) has published its 12th recommendation to the European Commission, proposing that four substances be added to the REACH Authorisation List (Annex XIV). This marks the first recommendation in 2.5 years, signaling a renewed focus on authorisation after a long pause.
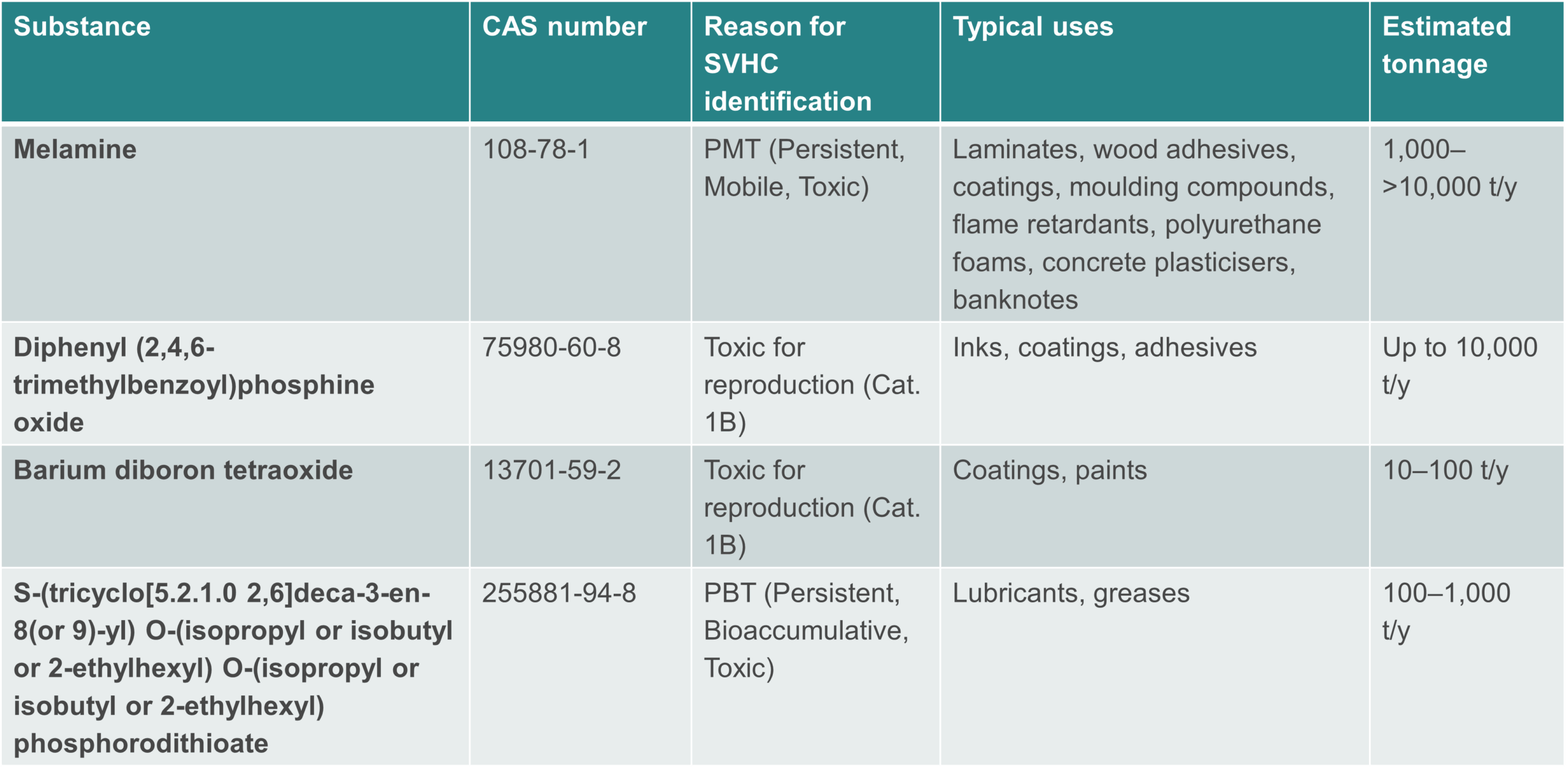
The four substances recommended to become subject to authorisation:
Why this matters
While these substances are not yet subject to authorisation, they have moved one step closer. The European Commission will now decide whether to include them in Annex XIV and set:
- A Latest Application Date (LAD) – the deadline to apply for authorisation
- A Sunset Date – the date after which use without authorisation is prohibited
- Any substance-specific exemptions
Businesses using these substances should act now to avoid compliance risks.
What’s unusual about this recommendation round? Spotlight on melamine
This is ECHA’s first recommendation for over two and a half years. ECHA paused new Annex XIV recommendations largely to provide headroom to manage chromium (VI) authorisations. The recent shift to restricting Cr(VI) under Annex XVII suggests ECHA is ready to resume its core authorisation work.
Melamine’s inclusion is notable. Industry challenged its identification as a substance of very high concern (SVHC) for its persistent, mobile and toxic (PMT) properties and its inclusion in Annex XIV, arguing that authorisation would create disproportionate burdens and major economic impact for downstream users. Melamine is a high-volume substance, used in laminates, coatings, adhesives, foams and more. While intermediate uses are exempt, downstream applications could face authorisation requirements, affecting furniture, construction, automotive, and electronics supply chains.
ECHA appears to have pushed ahead anyway, citing concerns over melamine’s PMT properties and potential for long-range water contamination. Melamine may be the first of many PMT or very Persistent very Mobile (vPvM) chemicals targeted for authorisation.
A snapshot of other substances:
- Diphenyl (2,4,6-trimethylbenzoyl)phosphine oxide (TPO): Common in inks and coatings; relatively high tonnage
- Barium diboron tetraoxide (barium metaborate, BMBF): Used in specialty coatings; lower tonnage
- S-(tricyclo[5.2.1.0 2,6]deca-3-en-8(or 9)-yl) O-(isopropyl or isobutyl or 2-ethylhexyl) O-(isopropyl or isobutyl or 2-ethylhexyl) phosphorodithioate: Used in lubricants and greases; moderate tonnage
What should businesses do now?
- Map – find these substances (or mixtures/articles containing them) in your supply chain
- Assess – determine if uses benefit from general exemptions (e.g. intermediate uses, low concentration)
- Strategise – prepare to substitute and/or for authorisation
- Communicate – work with suppliers and customers to assess options and manage risk
- Monitor – the European Commission may decide to include these substances in Annex XIV at any time; sunset dates and latest application deadlines will then define compliance timelines
Signals for the future?
A focus on PMT and very Persistent very Mobile (vPvM) substances under authorisation as well as restriction seems certain. PMT/vPvM evaluation is ongoing by ECHA’s RAC and PBT expert group, with priority given to substances flagged in REACH dossiers and drinking water contamination studies. ECHA and the German UBA estimate around 250–350 substances are strong candidates for PMT/vPvM classification based on current screening. These include pharmaceuticals, PFAS, pesticide metabolites, and industrial chemicals with high persistence and mobility.
Need help with REACH authorisation?
Sagentia Regulatory’s team can help you with all aspects of REACH authorisation:
- Screen your uses and supply chains to assess your vulnerability to these developments
- Prepare authorisation strategies
- Navigate exemptions and identify potential alternatives
- Assess PMT/vPvM classification
Read more of our advisories

December 16, 2025
EFSA’s role in the evaluation and integration of Codex MRLs into EU legislation and what it means for market access

November 21, 2025
ECHA recommends four new substances for REACH authorisation, including melamine. What does this mean for industry?
