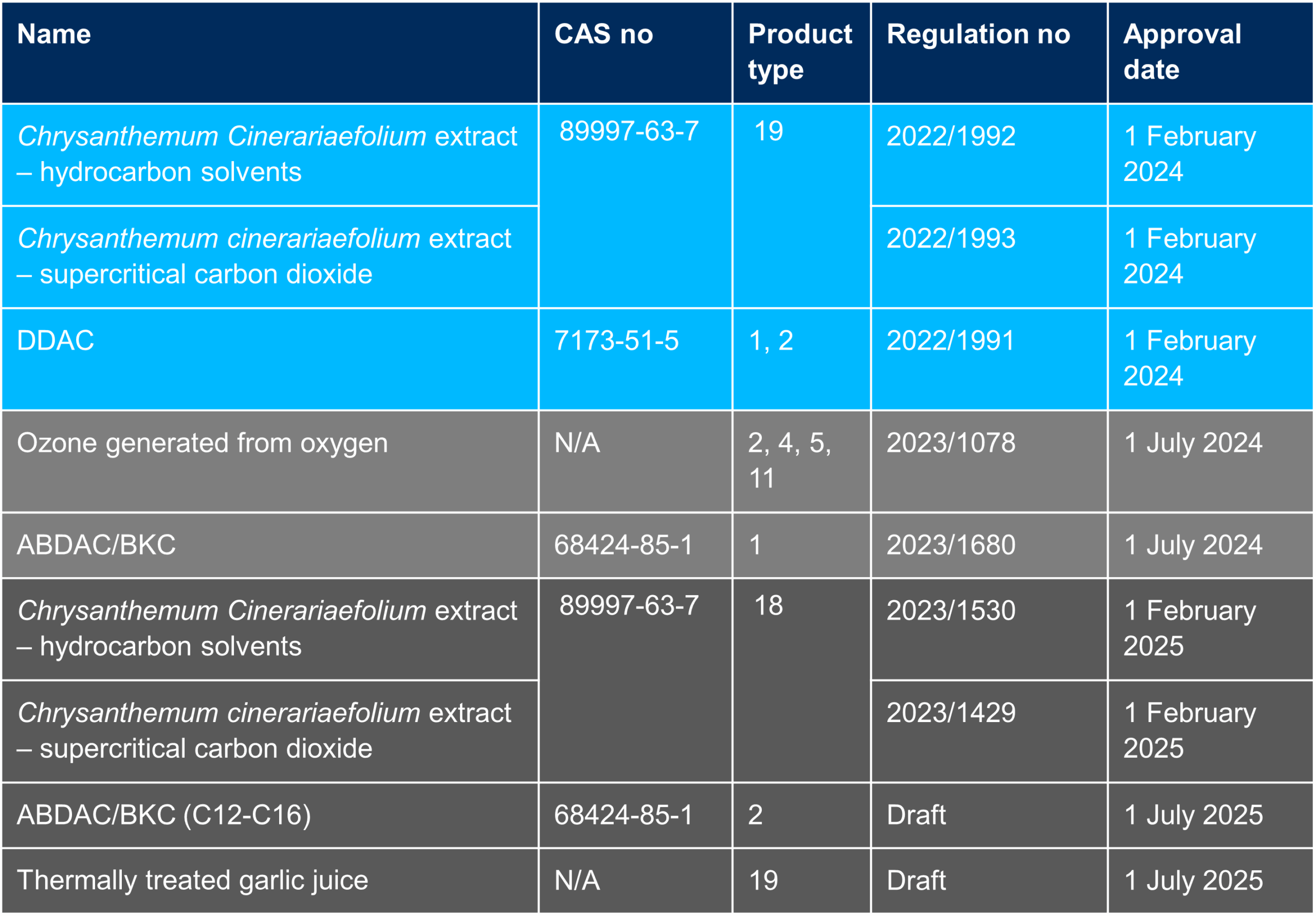
As a reminder, the review programme has already been extended twice. Initially under the Biocide Product Directive (BDP), the evaluation of active substances was set to be finished on 14 May 2010. This duration was extended by 4 years until 14 May 2014. With the BPR, the deadline became 31 December 2024 (Article 89.1). To date less than 45% of the actives have been reviewed.
The extension of this deadline has an impact on current national authorisations under transitional measures valid until 31 December 2024 and actions may be required in some member states. This is the case in Belgium where the authorities have already informed transitional authorisation holders about renewal/prolongation dossier submission deadlines.